*Source from beikecelltherapy and precedenceresearch.
Different Types of Stem Cells & Function of Stem Cells


Stay Eternal Youth with RegenCell


RegenCell Global as a distributor of stem cell products, our regulatory responsibilities are distinct from those of the manufacturers, yet equally critical in ensuring compliance across the supply chain. While all aspects of product development — including sourcing, processing, packaging, shipping and certification — are handled by our GMP-certified manufacturing partners, we maintain strict oversight of the regulatory status of each product we distribute. We ensure that all manufacturers adhere to relevant local and international regulations, including those set by agencies such as the FDA, EMA, or regional health authorities, depending on the market of distribution. This includes verifying that products are approved or registered where required, properly labeled, and accompanied by all necessary documentation such as Certificates of Analysis, Material Safety Data Sheets (MSDS), and batch tracking records. Our role as a distributor includes maintaining compliance with import/export regulations, cold chain logistics, and traceability standards, as well as ensuring that our operations align with best practices in product handling, storage, and distribution.
By working closely with our manufacturers and regulatory consultants, we help bridge innovative stem cell technologies with the clinical and research markets, all while upholding the highest standards of legal and ethical
RegenCell Global Corp. strictly adheres to all regulatory requirements set forth by the FDA, GMP, and ISO standards. Our products are sourced from manufacturers who follow the highest ethical standards in stem cell collection, processing, and storage. We provide complete traceability for every product we distribute, ensuring that each one meets the rigorous safety and quality guidelines set by governing bodies.”
All our products are manufactured in FDA-registered facilities and comply with GMP and ISO 13485 certifications. We work closely with trusted manufacturers who meet these exacting standards to ensure the highest quality stem cell therapies.
Our stem cell products are transported using temperature-controlled logistics systems, ensuring that every product maintains its integrity from point of origin to final destination. We adhere to strict cold-chain management procedures, maintaining optimal temperatures throughout the transport process to preserve the quality of the stem cells.
Note: RegenCell Global as a specialized distributor of stem cell products, we operate with a strong commitment to regulatory and ethical compliance across every step of our distribution process. While manufacturing, certification, packaging, and shipping are the responsibility of our qualified partners, we play a vital role in ensuring that all products we distribute meet applicable regulatory, legal, and ethical standards in their respective markets. We conduct thorough due diligence on all manufacturers to confirm compliance with Good Manufacturing Practices (GMP), applicable regenerative medicine regulations, and relevant certifications such as FDA registration, CE marking, or other country-specific approvals. Our compliance responsibilities also include ensuring accurate product labeling, maintaining full traceability and batch tracking, and adhering to cold chain and storage requirements as specified by the product’s regulatory guidelines. In addition, we comply with import/export regulations, maintain clear documentation, and ensure that our internal procedures align with best practices for quality assurance, risk mitigation, and data integrity. By working closely with manufacturers, regulatory consultants, and our clients, we ensure that all distributed stem cell products are handled responsibly, ethically, and in full alignment with national and international compliance frameworks.
In addition to distributing high-quality stem cell products, RegenCell Global Corp. offers a range of services designed to support healthcare providers, clinics, and research institutions. Our services ensure the safe and compliant use of stem cells, from product selection to delivery.
At RegenCell Global Corp., we act as a strategic distributor connecting licensed healthcare providers, clinics, and research institutions with trusted manufacturers of high-quality stem cell and regenerative products.
We manage the full distribution process for stem cell products, ensuring timely and reliable delivery to clinics, hospitals, and research labs. Our cold-chain logistics ensure that products are stored and transported at the correct temperatures to preserve their efficacy.
Note: RegenCell Global Corp. does not manufacture, alter, or ship any products directly. All products are shipped from and regulated by our certified manufacturing partners. Our mission is to simplify access and communication so you can focus on delivering care and innovation. While manufacturing, certification, and shipping are fully handled by our FDA-registered, GMP-certified manufacturing partners, our role is to ensure seamless access, reliable coordination, and clear communication between your clinic and the source.
Our team of experts provides regulatory consulting to ensure compliance with FDA and other regulatory bodies. We assist clinics and research institutions with the documentation, certifications, and steps required to integrate stem cells into their practices.
Our customer service team is available to answer questions, provide guidance on product usage, and resolve any issues quickly. We are here to support you at every step of the process.
Our services include:
We help you access premium biologic products from vetted, compliant manufacturers — including stem cell-derived therapies, cytokines, EVs, and more.
We coordinate with manufacturers to ensure you receive all necessary documentation — such as Certificates of Analysis (COAs), sterility reports, donor eligibility, and product specifications.
Our team provides guidance on product options, usage types, and clinical applications to help you make informed decisions for your practice or research.
We streamline the connection between providers and manufacturers, ensuring you have the information, support, and reliability you need — without the burden of navigating complex supply channels on your own.
At RegenCell Global Corp., our role is to bridge the gap between trusted stem cell manufacturers and licensed clinics, wellness providers, and research institutions. We do not manufacture or ship products ourselves — instead, we focus on streamlining access, ensuring transparency, and supporting your product sourcing requires from start to finish.
Here’s how our distribution process works:
Submit a product request or consultation through our team. Let us know your specific clinical or research needs.
Based on your needs, we recommend products from our network of FDA-registered, GMP-certified manufacturers, including stem cell-derived therapies, cytokines, EVs, and more.
All logistics — including product preparation, cold-chain shipping, regulatory certification, and documentation (COA, sterility reports, etc.) — are managed directly by the manufacturer.
The product is shipped directly to your authorized facility, along with full compliance paperwork and support for integration into your protocols.
RegenCell Global stays involved for support, follow-up, and communication — ensuring that your experience is smooth, compliant, and supported at every step.
⚠ Note: RegenCell Global Corp. does not handle manufacturing, storage, or shipment of any biologics. All products are processed, tested, and delivered by certified partners.
Because in regenerative medicine, trust matters.
At RegenCell Global Corp., we connect you with premium, ready-to-use stem cell products — ethically sourced, lab-tested, and fully backed by FDA-registered, GMP-certified manufacturers. We don’t just distribute biologics — we deliver peace of mind.
Top-tier Quality
We only work with manufacturers who meet the highest global standards.
Regulatory-Ready
Products come with full certification, documentation, and traceability.
Expert Support
We know the science — and we speak your language.
Whether you’re a clinician, wellness provider, or research leader, RegenCell helps you move forward — safely, confidently, and compliantly
At RegenCell Global Corp., we go beyond product supply — we deliver trust, transparency, and transformative potential. As a dedicated distributor of cutting-edge stem cell and regenerative products, we carefully select manufacturing partners who meet the highest global standards, including FDA registration, GMP compliance, and rigorous quality testing. Our role is to ensure a seamless connection between world-class manufacturers and frontline clinical providers — without delays, confusion, or risk.
What sets us apart is our commitment to integrity, regulatory excellence, and client support. We provide detailed product documentation, ongoing education, and regulatory guidance to help your clinic or research institution stay compliant and confident. Whether you’re a physician, clinic, biotech startup, or research lab, RegenCell delivers reliable biologics — and the professional support to use them responsibly.
Choose RegenCell Global Corp. for:
Why Choose RegenCell Global?
555 Anton Blvd, Suite #150
Costa Mesa, CA 92708
USA
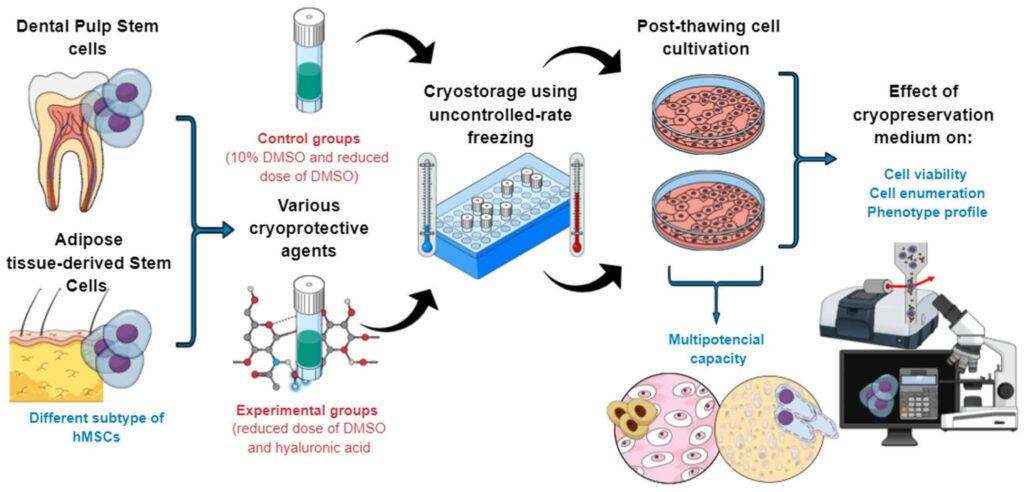
Stem cell therapy, a rapidly growing field in regenerative medicine, has shown incredible promise in the treatment of numerous diseases. Current research has led to groundbreaking advancements, such as the creation of organoids to study disease models and the potential for personalized medicine.
Researchers predict the advent of sophisticated techniques for stem cell manipulation, enabling the treatment of currently incurable diseases.
With advancements in regenerative medicine, stem cell therapy has gained considerable attention worldwide. Technological breakthroughs have improved the efficiency of stem cells in treating various diseases, from cancers to degenerative disorders. Yet, the therapy’s full potential is far from being fully realized.
Stem cell therapy, also known as regenerative medicine, promotes the reparative response of diseased, dysfunctional, or injured tissue using stem cells
Extraction process
involves isolating stem cells from the chosen source. Post extraction, the cells are cultivated in a controlled environment to increase their numbers. They are then transfused, or transplanted, into the patient’s body to replace damaged cells or to stimulate the body’s own repair mechanisms.
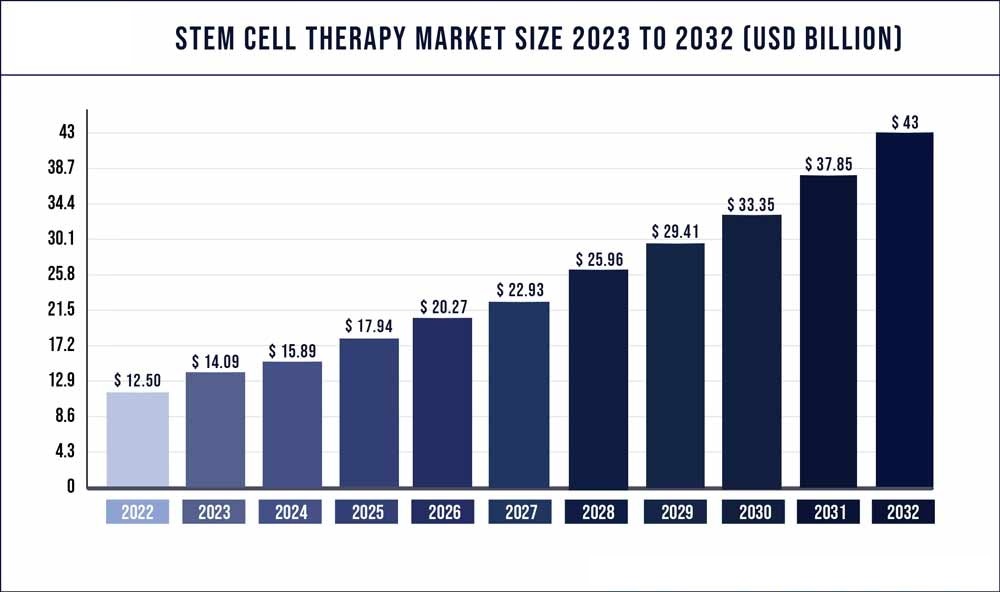
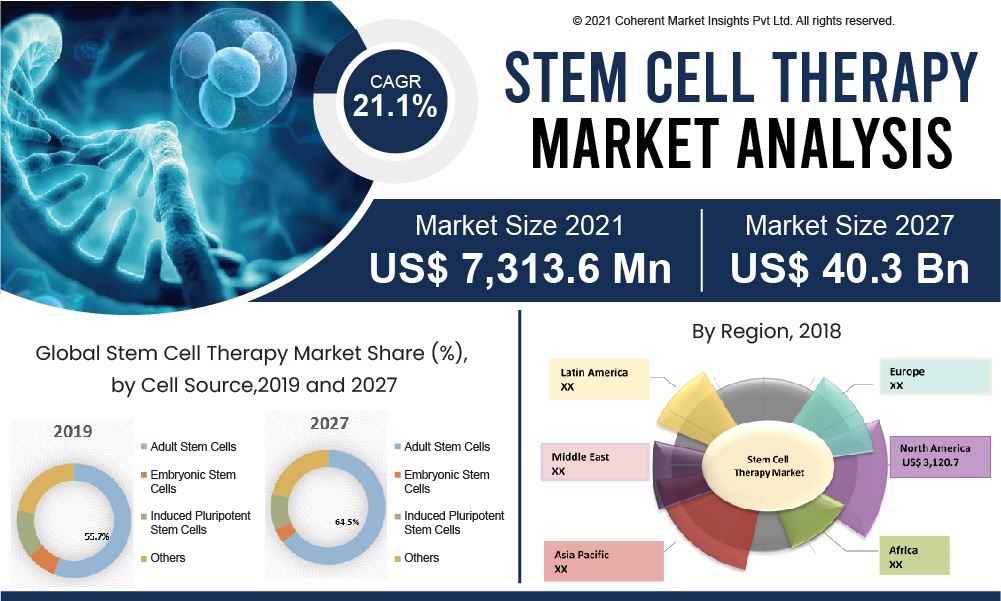
The global stem cell market size was valued at USD 9.6 billion in 2020 and is expected to grow in the coming years.
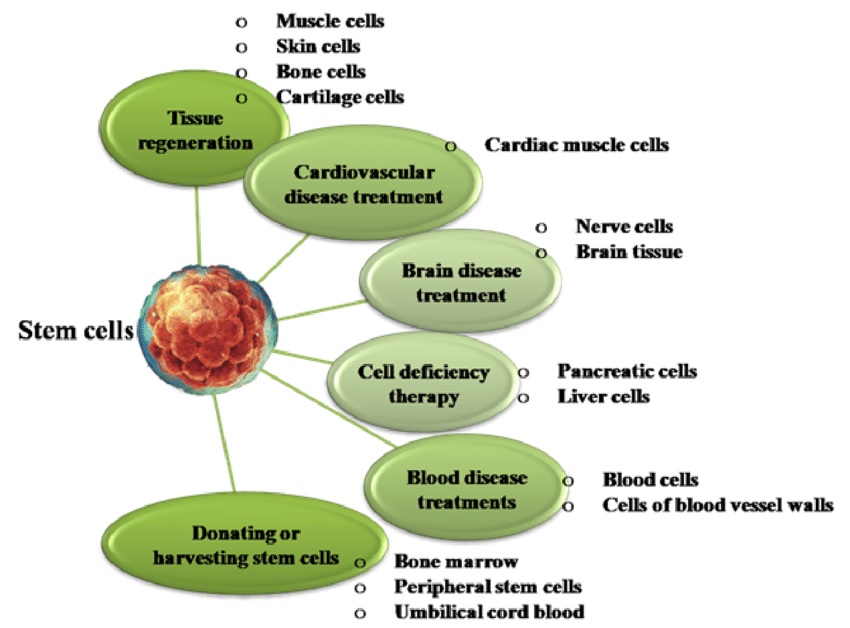
Applications of Stem Cell Therapy
Stem cell therapy, hailed as the future of medicine, holds immense promise in treating a range of diseases, including neurodegenerative disorders, spinal cord injuries, diabetes, heart diseases, cancers, etc. These cells, unique in their ability to self-renew and differentiate into other cell types, can replace damaged cells, thereby aiding in the treatment of these diseases.
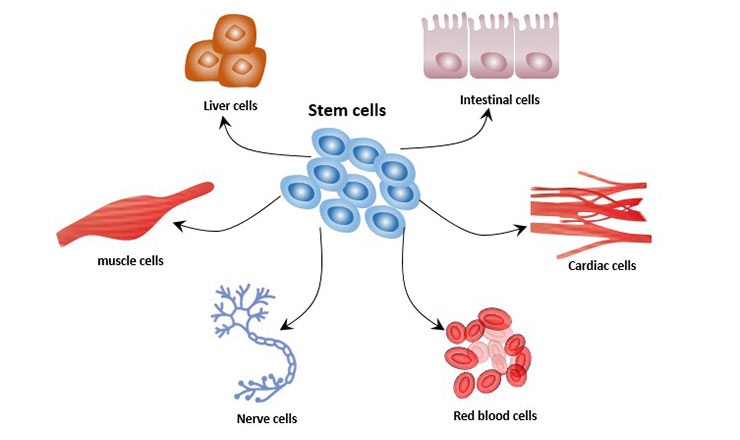
Stem cells play a critical role in the body’s healing process. Their regenerative capabilities allow them to replace damaged or diseased cells, restoring the normal function of the affected area. This ability has been leveraged by scientists to develop innovative treatments for previously incurable conditions.
At RegenCell Global Corp., we offer a comprehensive range of stem cell products that serve clinical and cosmetic applications. Whether you are a medical professional seeking solutions for regenerative therapies or a cosmetic clinic interested in anti-aging treatments, we deliver stem cell products that are ethically sourced, FDA-compliant, and rigorously tested for safety and efficacy.
Clinical Applications:
Our clinical stem cell products support a range of regenerative treatments, including tissue repair, immune system modulation, and recovery from injuries. Ideal for applications in orthopedics, neurology, cardiology, and more.
Cosmetic/Wellness Applications:
Our cosmetic stem cell products are designed for rejuvenation, skin healing, and hair restoration. These products are used in aesthetic clinics and wellness centers to promote anti-aging, reduce wrinkles, and stimulate collagen production.
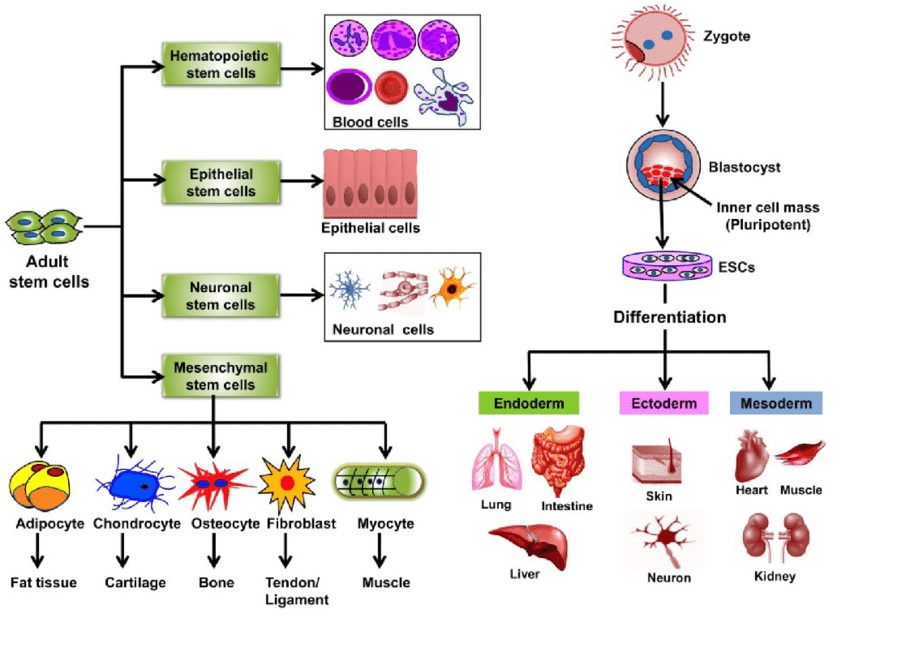
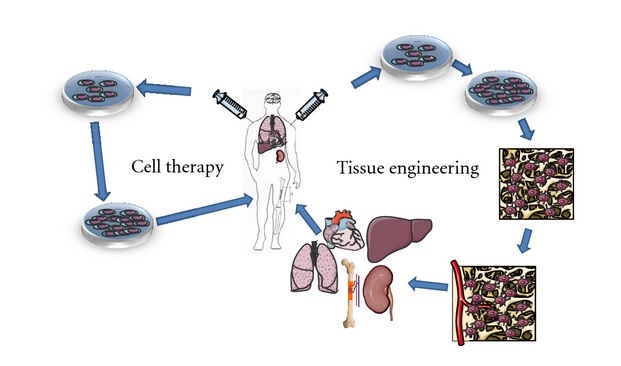
Stem cell therapy, also known as regenerative medicine, is a revolutionary scientific and medical approach that focuses on restoring the function of damaged or diseased tissues or organs. It involves the use of stem cells, which have the unique ability to develop into different cell types in the body.
*Source from beikecelltherapy and precedenceresearch.
Since the first successful bone marrow transplant in 1956, the field of stem cell therapy has evolved significantly. Its potential applications in healthcare are vast, ranging from treating various diseases like cancer and diabetes to repairing damage caused by injuries.
We distribute high-quality stem cells sourced from:
Benefits:
Extracellular Vesicles (EVs), including exosomes, are nano-sized particles secreted by stem cells. They contain proteins, lipids, RNA, and other bioactive molecules that mimic the therapeutic effects of stem cells—without the cells themselves.
Applications:
Benefits:
Contains mesenchymal stem cells ideal for regenerative therapies Umbilical cord-derived mesenchymal stem cells (UC-MSCs) are collected non-invasively from the Wharton’s Jelly of postnatal umbilical cords. These cells are young, immune-privileged, and have high regenerative capacity.
Applications:
Benefits:
Cytosomes are proprietary, cell-derived nanoparticles engineered for targeted delivery of biologically active substances. Think of them as next-gen exosomes, optimized for specific therapeutic targets.
Applications:
Benefits:
Placental tissue and fluids are rich in growth factors, stem cells, cytokines, and extracellular matrix components, making them ideal for regenerative therapies. Our placenta-derived products are ethically sourced from full-term, healthy births.
Applications:
Benefits:
Amniotic fluid contains a potent mixture of growth factors, hyaluronic acid, cytokines, and extracellular vesicles. Our purified, non-cellular amniotic fluid products are cell-free but biologically active, offering strong therapeutic effects without the regulatory complexity of live cells.
Applications:
Benefits:
Bone marrow-derived stem cells are a highly versatile and well-studied type of adult stem cell, harvested from the spongy tissue inside bones—most commonly the iliac crest (pelvic bone). These stem cells include both mesenchymal stem cells (MSCs), which support tissue repair and regeneration, and hematopoietic stem cells (HSCs), which give rise to blood and immune cells.
Our BM-MSCs are clinically prepared, either for autologous use (from the patient) or allogeneic use (from screened donors), depending on your therapeutic model.
Applications:
Benefits:
These stem cells, sourced from adipose (fat) tissue, have the ability to regenerate and repair tissues. They are used in a variety of clinical applications, including wound healing and joint regeneration.”
Potent and abundant type of adult mesenchymal stem cell (MSC) harvested from adipose (fat) tissue. Due to their ease of collection and rich concentration of regenerative factors, ADSCs are one of the most widely used stem cell types in clinical, aesthetic, and regenerative medicine.
Collected via minimally invasive liposuction procedures, ADSCs demonstrate powerful abilities to differentiate, modulate inflammation, and accelerate tissue healing.
Applications:
Benefits:
RegenCell Global Corp. operates as a specialized distributor of high-quality stem cell products, serving the clinical, cosmetic, and wellness sectors. We collaborate with a network of trusted, FDA-registered, GMP-certified manufacturers to provide a reliable supply of biologics to clinics, hospitals, and research institutions.
While we do not directly produce or ship the products, all manufacturing, quality control, certification, and logistics are managed by our manufacturing partners. These partners are fully responsible for:
RegenCell Global ensures smooth coordination between providers and manufacturers, offering product education, regulatory documentation support, and client service to ensure a seamless experience from inquiry to delivery.
Founded in March 2025, RegenCell Global Corp. emerged from a vision to bridge the gap between groundbreaking stem cell technologies and those who can benefit from them. With decades of combined experience in the healthcare and biotech industries, our team saw the potential of stem cell therapies in regenerative medicine and wellness. As the industry grows and more clinics, hospitals, and research institutions seek reliable sources of stem cell products, we aim to be their trusted partner. By ensuring that every product we distribute meets the highest standards of quality, safety, and compliance, we are proud to be a leader in the evolving field of stem cell therapies. We supply the world with high-quality stem cells that support life-changing innovation.
We work with certified laboratories and adhere to strict quality standards to ensure every product is ethically sourced, thoroughly tested, and ready for clinical or research. We believe in the future of regenerative therapies. Our mission is to empower research and clinical innovations by providing top-tier, ethically acquired stem cell products.
Mission
To provide healthcare professionals with top-tier stem cell products, enabling them to offer innovative regenerative therapies and cosmetic solutions that improve patients’ lives
Vision
To become the most trusted distributor of stem cell therapies globally, helping to make regenerative treatments accessible to clinics, hospitals, and research institutions while adhering to the highest standards of safety and compliance.
Our Team
RegenCell Global’s executive management are:
Mr. Calvin Cao is a founder of RegenCell Global Corp. As an inventor, innovator and Bio-Entrepreneur, his strategy and commitment to make RegenCell Global the dominant global company on the market for the entire field of cell therapy. In 2008, as a chairman and co-founder of Stem Cell Therapy International Corp. He engineered the merger of Stem Cell Therapy International and Histostem of South Korea. Histostem, one of the largest fully accredited cord blood banks in the world, with more than 80,000 cord blood units for use in research and treatments. The successful merger has formed one of the first fully merged Pacific Rim stem cell companies and cord blood repositories with a U.S. entity Amstem Corp.
He graduated his doctorate of Medicine in France – Faculté de Médecine Paris-Descartes. He is obtained his PhD of Science Economics and his MBA of Strategy & Management. He was a financial manager and businessman working in the banking and financial domain for more than 30 years. There was also high level advisor-consultant in the banking and financial institutions, on the strategy, the change and the commercial development.
Welcome to RegenCell Global Corp.— your trusted partner in regenerative medicine. We are a premium distributor of ethically sourced stem cells, advancing the frontier of regenerative medicine and clinically validated stem cells for research and therapeutic.
We deliver high-quality biological products to researchers, clinicians, and institutions worldwide
RegenCell Global Corp. is a leading distributor of high-quality stem cell products, dedicated to supporting the healthcare and wellness industries. We connect cutting-edge stem cell manufacturers with medical clinics, hospitals, biotech companies, and research institutions across the United States. Our role is to ensure seamless product distribution, compliance with stringent regulatory standards, and the highest level of customer satisfaction. With a focus on both clinical applications, including regenerative therapies for orthopedic, neurological, and cardiology treatments, and cosmetic wellness solutions, we are committed to advancing the accessibility of stem cell-based treatments. Whether you’re a healthcare provider or a researcher, RegenCell Global Corp. delivers reliable, compliant, and safe stem cell solutions.